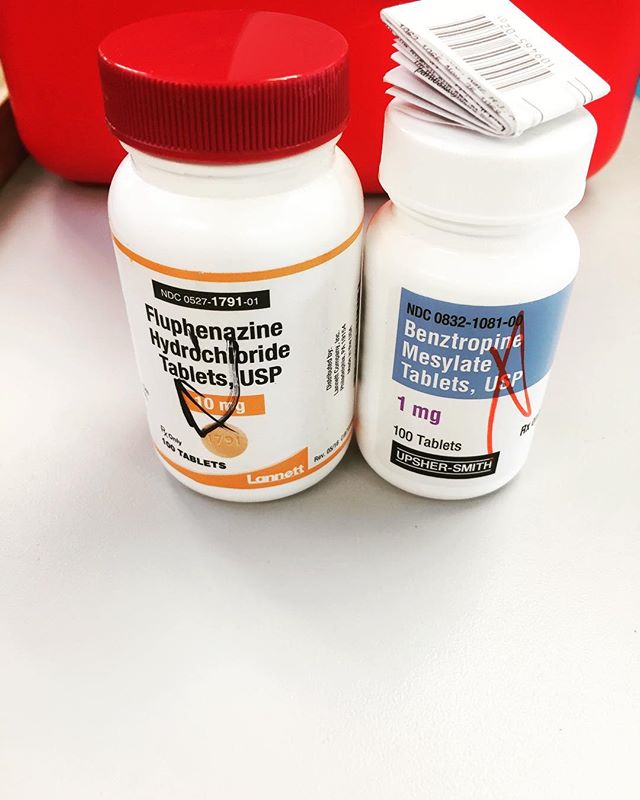
What is a DUR?
/As a pharmacist, I perform drug utilization reviews (DURs) every single day. I felt that I had a pretty solid understanding of what they encompass, but while doing the research for this post I learned that there are many, many, different definitions.
The most comprehensive definition I found during my research is from the Academy of Managed Care Pharmacy (AMCP); they define drug utilization review (DUR) as “an authorized, structured, ongoing review of prescribing, dispensing and use of medication.” The AMCP (and many others that have written about DURs) references 3 distinct categories: prospective, concurrent, and retrospective. There is a wealth of information describing the intricacies of the categories, and I learned a great deal as I was putting together this post. If you are interested, and have the time, I definitely recommend reading through a few of the articles available. For the purposes of this post, I will focus on the category of DUR that is most common in the retail pharmacy space, the prospective drug utilization review.
There are a few different definitions of prospective DUR, but they all essentially convey the same message. Essentially, this is a review that examines various drug therapy issues and occurs before the patient receives the medication. In the retail pharmacy setting, this is usually performed by the pharmacist, utilizing clinical knowledge paired with alerts from their pharmacy software. First Data Bank is the provider of this software for the majority of retail pharmacies in the United States.
There are countless issues that may arise during this process, but the most common are drug-drug interactions, drug-disease interactions, dosing issues, and drug-patient precautions (such as pregnancy/nursing status, pediatric and geriatric dosing/contraindications, drug allergies, gender, etc.). These alerts occur frequently (some argue too frequently), and it is imperative that pharmacists are able to evaluate them appropriately and make the necessary interventions to ensure both patient safety as well as the best possible clinical outcomes. There has been increased scrutiny of this process recently after an article was published in the Chicago Tribune. I wrote my thoughts on the article in a separate post.
The concept of DURs seems to have originated with the Omnibus Budget Reconciliation Act of 1990 (OBRA 90). This legislation was created to reduce the federal budget deficit and was signed into law by President George Bush in November of 1990. The Act addressed a multitude of budget issues and contained a section outlining changes to Medicare, Medicaid and other health related programs. If you are interested in reading the full text of the Act, I have linked it here. OBRA 90 was also responsible for creating many of the guidelines pertaining to patient counseling that we adhere to today.
Performing a drug utilization review is one of the most important roles of a community pharmacist. Since so much of what I do clinically in retail pharmacy involves DURs, I feel that it is beneficial to write about specific situations I have encountered and how I choose to handle them. There are definitely many methods and schools of thought and I’m hoping to get some reader feedback and be able to include some teaching points and clinical pearls. Please check out the ones I have written about so far and check back for future posts.